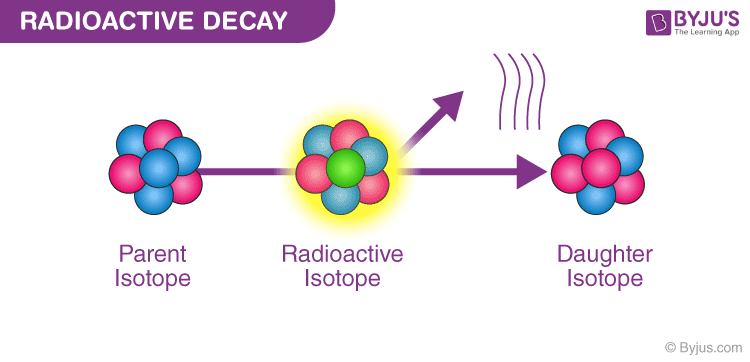
Some elements of atomic nuclei are unstable because of the presence of excess nuclear charge inside it, so these nuclei undergo radioactive decay to form stable nuclei. These elements are called radioactive elements.
The stability of nuclei of an element can be calculated by n/p (neutron to proton ratio). Elements of atomic number up to Z= 20 are stable and contain an equal number of protons and neutrons. As the atomic number increases, the repulsion between protons increases and requires more protons and neutrons. Thus, the neutron to proton ratios of stable nuclei increases with increasing atomic number.
Elements having atomic number up to Z = 20 , n/p =1.0 . And the elements having atomic number up to Z=83, n/p = 1.5 . If elements have the atomic number, Z > 83, n/p will be greater than 1.5 and it will be most unstable and hence radioactive.

When an alpha particle emits its nucleus, the process is called alpha decay. The formula of alpha decay is given as:
The nucleus of helium is taken as the alpha particle which is very stable. It has a group of two protons and two neutrons. For example, the alpha decay of uranium-238 is shown below
A beta particle is often referred to as an electron, but it can also be a positron. If the reaction involves electrons, the nucleus sheds out neutrons one by one. Even the proton number increases accordingly. A beta decay process is shown below:
The nucleus has orbiting electrons which indeed have some energy, and when an electron jumps from a level of high energy to a level of low energy, there is an emission of a photon. The same thing happens in the nucleus: whenever it rearranges into a lower energy level, a high-energy photon is shot out which is known as a gamma ray.
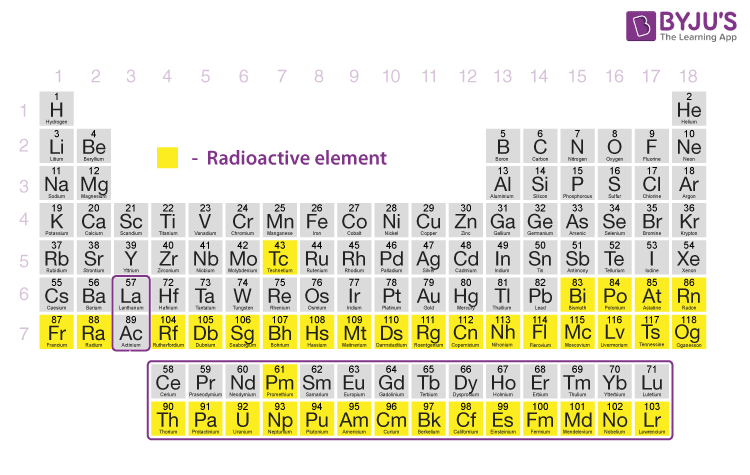
Here’s the list of radioactive elements and their most stable isotopes:
| Radioactive Elements | Atomic number | Most stable isotope |
|---|---|---|
| Technetium | 43 | Tc-97 |
| Promethium | 61 | Pm-145 |
| Polonium | 84 | Po-209 |
| Astatine | 85 | At-210 |
| Radon | 86 | Rn-222 |
| Francium | 87 | Fr-223 |
| Radium | 88 | Ra-226 |
| Actinium | 89 | Ac-227 |
| Thorium | 90 | Th-232 |
| Protactinium | 91 | Pa-231 |
| Uranium | 92 | U-235 |
| Neptunium | 93 | Np-237 |
| Plutonium | 94 | Pu-244 |
| Americium | 95 | Am-243 |
| Curium | 96 | Cm-248 |
| Berkelium | 97 | Bk-247 |
| Californium | 98 | Cf-251 |
| Einsteinium | 99 | Es-252 |
| Fermium | 100 | Fm-257 |
| Mendelevium | 101 | Md-258 |
| Nobelium | 102 | No-259 |
| Lawrencium | 103 | Lr-266 |
| Rutherfordium | 104 | Rf-267 |
| Dubnium | 105 | Db-268 |
| Seaborgium | 106 | Sg-269 |
| Bohrium | 107 | Bh-278 |
| Hassium | 108 | Hs-269 |
| Meitnerium | 109 | Mt – 282 |
| Darmstadtium | 110 | Ds-281 |
| Roentgenium | 111 | Rg-286 |
| Copernicium | 112 | Cn-285 |
| Nihonium | 113 | Nh-286 |
| Flerovium | 114 | Fl-290 |
| Moscovium | 115 | Mc-290 |
| Livermorium | 116 | Lv-293 |
| Tennessine | 117 | Ts-294 |
| Oganesson | 118 | Og-294 |
Some elements of atomic nuclei are unstable because of the presence of excess nuclear charge inside it so these nuclei undergo radioactive decay to form stable nuclei. These elements are called radioactive elements.
The common 4 radioactive elements are Uranium, Radium, Polonium, Thorium etc.
Cigarettes made from tobacco contain radioactive materials: polonium-210 and lead-210. These radioactive particles settle in smokers’ lungs, where they build up as long as the person smokes. Over time, the radiation can damage the lungs and can contribute to lung cancer. They also harm people exposed to secondhand smoke.
U-235 and U-238 occur naturally in nearly all rock, soil, and water. Uranium is now used to power commercial nuclear reactors that produce electricity and to produce isotopes used for medical, industrial, and defence purposes around the world.
Radioactive waste is a type of hazardous waste that contains radioactive material. Any activity related to the nuclear fuel cycle that produces or uses radioactive materials generates radioactive waste. The activities like mining and processing of uranium ore, fabrication of nuclear fuel, generation of power in nuclear reactors, production and use of radionuclides for various industrial and medical applications, research associated with radioactive material etc. generate the different types of radioactive waste.